

 Heart Valve repair and treatment
Heart Valve repair and treatment Congenital Heart Disease Treatment
Congenital Heart Disease Treatment
Basic information
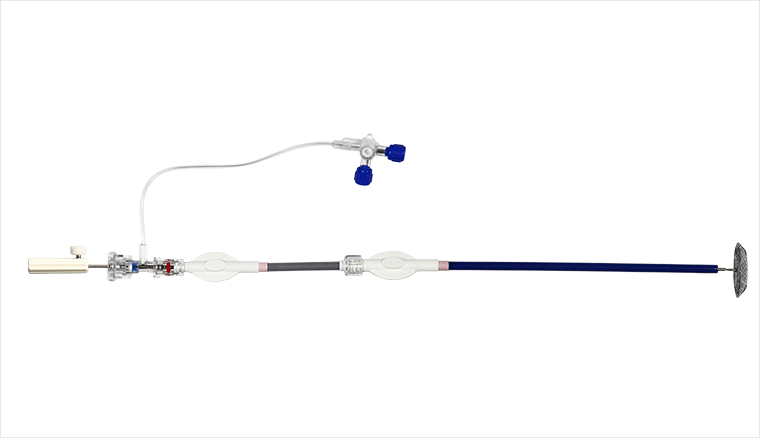
"Cardiovascular disease occluder delivery system" is a medical device designed and developed by our company based on market demand. This product is used as a delivery device for congenital cardiac septal defect occluders and is used to implement the delivery of occluders of our company. Clinical Use it in conjunction with the sealing device produced by our company. This product is divided into two types: medical type and surgical type according to the different delivery sheaths. There are seven specifications in total, namely 6F, 7F, 8F, 9F, 10F, 12F and 14F. This product first obtained the Medical Device Trial Production Registration Certificate in 2004, with the registration number being State Food and Drug Administration (Trial) No. 2004 No. 3061266, and most recently obtained the medical device renewal certificate from the State Food and Drug Administration in August 2015. Registration certificate, registration number is National Machinery Registration Approval 20153771494.
The cardiovascular occluder delivery system includes three parts: a long sheath, a short sheath (loader) and a steel cable. The long sheath includes a long sheath and an inner core (or called tube core). The proximal end of the long sheath tube has a joint that can be connected to the loader; the loader is a tool used to load the occluder into the long sheath and does not enter the body; the proximal end of the delivery steel cable is a handle for extracorporeal operation, and a screw is welded to the other end , the screw can be screwed with the screw cap of the occluder. Sterilized by ethylene oxide, the product is disposable.
|

|

|
|
|
|
Scope of application
The cardiovascular disease occluder delivery system (hereinafter referred to as the delivery system) is divided into atrial defect occluder delivery system and patent ductus arteriosus occluder delivery system, in which the atrial defect occluder delivery system is used for atrial defect occluder ( ASD), the patent ductus arteriosus occluder delivery system is used for the delivery of patent ductus arteriosus occluder (PDA) and ventricular septal defect occluder (VSD).