Heart Valve repair and treatment
Heart Valve repair and treatment Congenital Heart Disease Treatment
Congenital Heart Disease Treatment
Basic information
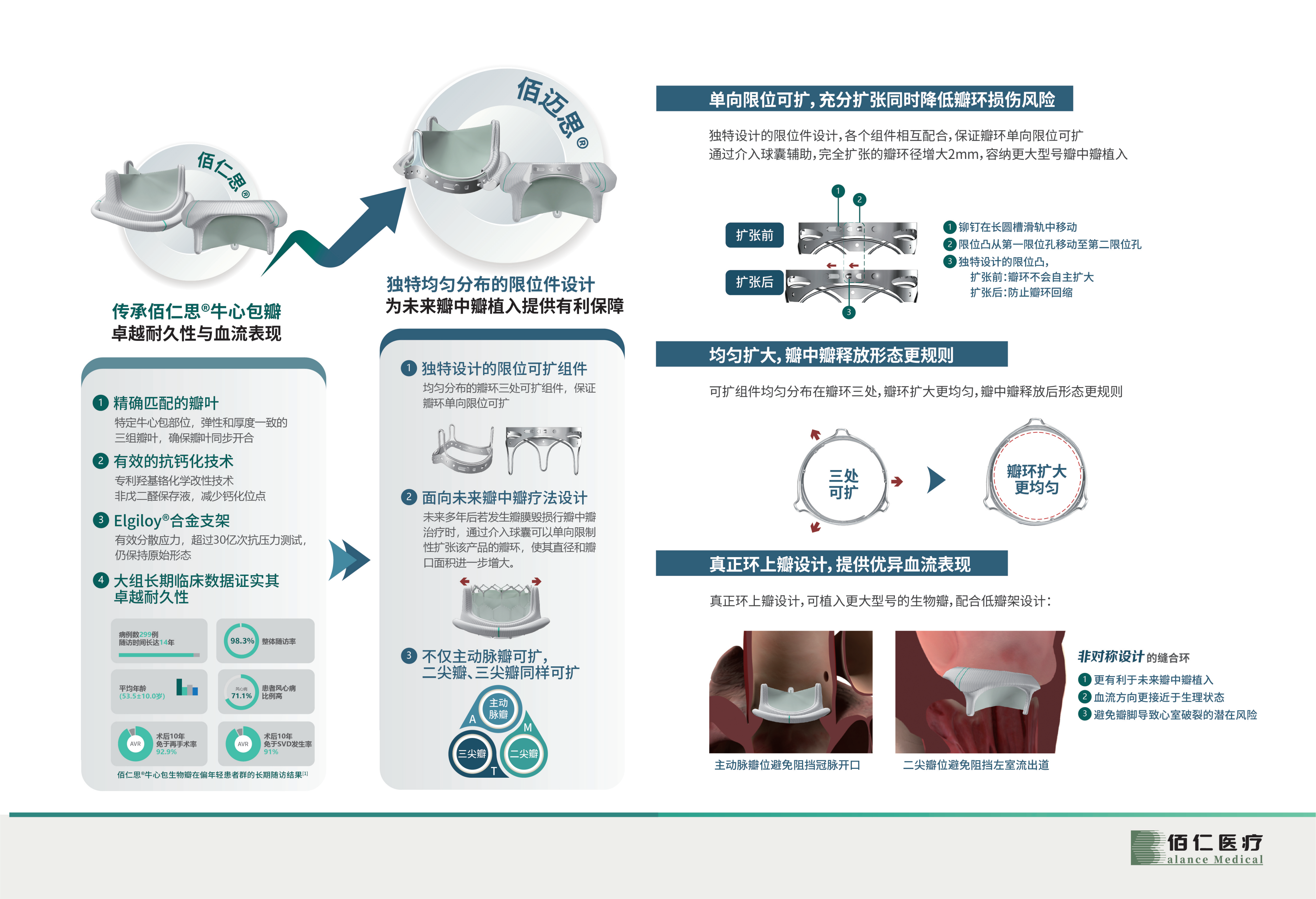
Recently, after review by the State Food and Drug Administration, the innovative product self-developed by Beijing Bairen Medical Technology Co., Ltd., the limited expandable artificial biological heart valve (National Medical Device Registration Approval No. 20233131137) was approved for registration. Compared with traditional surgical biological valves, This product provides more favorable protection for possible future transcatheter valve-in-valve treatments. The product's applicable scope covers the aortic valve, mitral valve, and tricuspid valve. It is the first domestically produced expandable surgical biological valve and the world's first marketed expandable surgical valve covering the treatment of mitral valve and tricuspid valve. Bioprosthetic valves fill a gap in the market.




Performance advantages
Scope of application
This product is used to replace diseased, damaged, and deformed aortic valves, mitral valves, and tricuspid valves, as well as previously implanted artificial aortic valves, mitral valves, and tricuspid valves.